In this article I report about my personal experience and share in the discovery and development of fluazuron (initially known as CGA-1574199) and ACATAK in the 1990s. For a better understanding it is useful to get a feeling on the background and frame where this took place, i.e. the people and the activities in Ciba-Geigy Animal Health in those years.
April 1985: joining Ciba-Geigy Animal Health
I joined Ciba-Geigy AH in April 1985. It was my first job in the industry. I had finished my PhD in Entomology in the Swiss Federal Institute of Technology (ETH) in Zürich, and had been working a few months in a research project on Lepidopteran spermatogenesis. At that time, my major motivation for getting a job in the industry was to obtain a work permit that would allow me to remain in Switzerland. As a Spaniard in Switzerland in 1985, the immigration authorities wanted me to leave Switzerland asap after I had finished my Phd. And the only way to get around it was to obtain a job there. This was not easy, because work permits for foreigners were quite difficult to get in those years, particularly for mid and small companies:  they had to proof that they had no adequate Swiss candidates. And being a Spaniard in Switzerland in 1984 (eight years before Spain joined the EU) was not precisely a competitive advantage.
they had to proof that they had no adequate Swiss candidates. And being a Spaniard in Switzerland in 1984 (eight years before Spain joined the EU) was not precisely a competitive advantage.
I had sent dozens of applications, and often got the answer that they were unable to get a work permit for a Spaniard. Ciba-Geigy was the only company that asked me for an interview. They were big enough to get a work permit for me or whoever else. After several meetings and interviews (including a graphological analysis) I finally got a job in the Centre de Recherches Agricoles (CRA), one of the three research stations of Basle-based Ciba-Geigy Animal Health in those years, the other two stations being Yarrandoo, close to Sydney in Australia, and Baynes Drift, close to Pietermaritzburg in South Africa.
I have to admit that coming from basic research in the highly reputated Swiss Polytechnical School, I rather looked down on applied research as practiced in the industry, a kind of class-B science for me in those years. However, the research center looked great. It had been built a decade before and was equipped with the latest technology available in those years. And St-Aubin was a really idyllic spot in the middle of Switzerland, very close to the lakes of Neuchatel and Murten.
My new team was running the Screening for the discovery and characterization of new active ingredients with parasiticidal potential. The best candidates were proposed for further development that was ensured by another team based in Basel. A few months after joining the team I realized that all my new colleagues were highly competent and experienced, very efficient, well organized, and very kind to me, the youngest colleague. The fact that I already spoke Swiss-German dialect fluently probably helped, because they were all Swiss-Germans. I am still grateful to all of them, and I want to thank them here for those wonderful years: Rolf Immler (the team leader), Walter Häusermann (my fist boss), Hans Bouvard, Max Maurer, Andreas Murbach, Jürg Strittmatter, and Markus von Orelli.
Besides our Screening Unit, there was an independent team of mostly English-speaking colleagues in the recently created BioVet Unit (relevant for my future development in Ciba-Geigy…). They were trying to develop new veterinary products out of some molecules licensed from Genentech (e.g. some kind of interferons).
My first job
My first responsibility consisted in managing the primary in-vitro screening for ectoparasiticides. When I took over we were testing about 300 new compounds a week for their efficacy against ticks, mites, adult flies and fly larvae. We had virtually zero computer support. Everything was written by hand: lists, and lists, and more lists… The only computer-like device in the whole center was a terminal plaved in an empty office and connected to the mainframe in Basle. We just fed the screening results into that terminal and hoped it would be useful to someone down the line. We had no printer either: if ever you wanted to print something it was physically done somewhere in Basle and you got it by internal mail a few days later. Sometimes you got a huge printout with thousands of pages: another wrong print order…
I was immediately nominated responsible for all computing in the unit: being the last one that joined the team and the youngest, I was supposed to be still adaptive enough for such a job, and to have time to learn how to do it. My senior colleagues had more important things to do. Around 1987 I bought the first PC in the center, one for the whole unit (a HP-Vectra).
One of my weekly tasks was to check which compounds had performed well in each screening tests, and to select them for the next screening level if the chemistry or the spectrum of activity were promising.
Pretty soon two new compounds attracted my attention: CGA-183893 and CGA-184699. “CGA” stood for Ciba-Geigy-Agro” and the numbers were sequentially allocated to new chemicals synthesized in the chemistry department. Both compounds were screened and discovered within about one month. Years later CGA-183893 was a to become dicyclanil, the active ingredient of CLIK®, nowadays the “Rolls-Royce” for blowfly strike prevention in Australia, New Zealand, UK and other countries. CGA-184699 was to become lufenuron, the active ingredient of PROGRAM®, the first once-a-month tablet against fleas in dogs. To learn about the discovery of these two compounds visit the respective articles in this site on the discoveries of dicyclanil (CLiK) and lufenuron (PROGRAM).
Ciba-Geigy Animal Health in 1985
It is useful to put the discovery of fluazuron in the context of Ciba-Geigy’s AH business with livestock parasiticides in those years. In the 60s and 70s Ciba-Geigy had been very successful in this market, mainly with organophosphates: diazinon (NEOCIDOL, TOPCLIP), dichlorvos (NUVAN), chlorfenvinphos (STELADONE) & azamethiphos (SNIP, ALFACRON, etc.). But cymiazole (TIFATOL, ECTOBAN), Ciba-Geigy's own amidine, supposed to re-enforce the tickicides portfolio had failed to compete with amitraz, basically because it had almost no residual effect. This meant that cattle had to be treated more frequently with cymiazol than with amitraz, the competitor to beat. Frustration in the team was significant, because cymiazole had been developed following the assumption that active ingredients with none or very short residual effect would be less susceptible to developing tick resistance and thus "better" than amitraz, an assumption vastly admitted and supported by most scientists in those years. But most cattle farmers thought differently, and cymiazole was a non-starter. Hence when CGA-157419 (fluazuron) was discovered many people in Ciba-Geigy said: finally a product with residual effect.
And more important, in the decade between 1975 and 1985 Ciba-Geigy had completely missed the synthetic pyrethroids. They had got access to cypermethrin (ECTOMIN) but this was not their baby, success was limited, and many other competitors had access to cypermethrin or other similar pyrethroids as well. And it could not compete with BAYTICOL (Bayer’s flumethrin) or BUTOX (Roussel Uclaf’s deltamethrin), market leaders in those days. As a consequence Ciba-Geigy was slowly but steadily losing market share and the pressure for a new tickicide had been very strong in the previous years.
Nevertheless, there were also recent success stories in Ciba-Geigy AH, e.g. cyromazine, a very effective, innovative and successful larvicide against houseflies (LARVADEX, NEPOREX) and blowfly strike on sheep (VETRAZIN); and triclabendazole, (FASINEX, ENDEX), the best flukicide for sheep and cattle ever developed, still market leader today.
One strategic weakness of Ciba-Geigy AH in those years was that it was almost absent from the US, the largest market, and very weak in Japan, the third market after the EU. The management was aware and was pushing the entry into these two markets. For any new project the so-called TRIAD potential was essential, i.e. the potential in the US, the EU and Japan.
The launch of MSD’s ivermectin (mythic IVOMEC) in the early 1980s surprised and impressed most Animal Health people worldwide, including those in Ciba-Geigy. Employees’ gossip I was unable to confirm said that avermectins had been initially offered to Ciba-Geigy but had been rejected by the management because they were already engaged with SANKYO’s milbemycins. In fact we were heavily screening hundreds of milbemycin analogues, but nothing comparable to ivermectin had been found yet, and was never discovered later on. Milbemycin oxime (INTERCEPTOR, SENTINEL, MILBEMAX) was discovered and developed in those years, but only for pets. It was handled by our endoparasiticides screening, where I was not directly involved initially.
In 1985 the companion animal business of Ciba-Geigy was pretty irrelevant, with basically no significant ectoparasiticidal product against fleas or ticks. But the management had already decided to strengthen research into this market, because such products fitted best in the TRIAD-strategy previously mentioned. This was to affect fluazuron’s future significantly…
CGA-157419 discovery: enthusiasm
But let’s come back to fluazuron. When I joined Ciba-Geigy in 1985 it was still known as CGA-157419. It had been discovered about two years before. Based on very promising results in various tests with Rhipicephalus (Boophilus) microplus (the cattle tick) and Dermanyssus gallinae (the red fowl mite) it had just started pre-clinical development. First in vivo results suggested a significant potential against many tick and mite parasites of livestock and pets. My boss, Walter Häusermann, was particularly proud of having "beaten" BAYER’s screening with CGA-157419. He knew that BAYER had also screened the same molecule but have missed it. From a previous courtesy visit to BAYER’s research center in Mohnheim he knew that their screening test with engorged female ticks was routinely stopped before hatching of the oviposited eggs, whereas our own test waited until egg hatching. And in such a test the efficacy of CGA-157419 consisted precisely in inhibiting egg hatching.
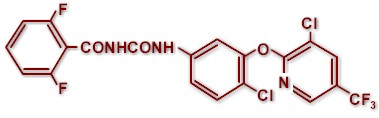
CGA-157419 is a benzoylphenyl urea (BPU), a well-known chemical class of Chitin Synthesis Inhibitors. The first BPU that came to market in 1975 was diflubenzuron (from Philips-Duphar B.V.). As usual in all companies in such cases, Ciba-Geigy’s chemists synthesized dozens if not hundreds of diflubenzuron analogues that were tested in the screening. The immense majority showed more or less strong efficacy against insect larvae, but none against ticks. CGA-157419 was the best compound of a group of BPU’s that behave differently: they were very effective against ticks and mites, but not at all against insects, or only at unpractical concentrations.
Particularly promising was the fact that besides contact effect, CGA-157419 had a systemic effect against ticks as well: it worked also through the host’s blood. First in-vivo trials on cattle had shown that protection against re-infestations of up to 12 weeks could be achieved. This was a tremendous progress towards our benchmark, Bayer’s BAYTICOL (with flumethrin), with a claim of up to 6 weeks protection. And being a Tick Development Inhibitor CGA-157419 was virtually harmless to mammals, birds and fish, because it acted on chitin synthesis, a biochemical system that does not occur in vertebrates.
Walter, my boss, was enthusiastic and very efficiently running the whole test program. CGA-157419 was not my project, but I shared the enthusiasm with him and followed its progress. About two years later CGA-157419 was officially selected for development. In those years in Ciba-Geigy this meant that a so-called New Product Team, the NPT, took responsibility for further activities. The NPT was made of two specialists, one for Clinical Development and one for Marketing, and was based in the Basle headquarters, not in St-Aubin. Hence CGA-157419 “left” our Research Center in St-Aubin to be managed directly from Basel.
Fluazuron’s early development: disappointments and threats
Although no more on the driver’s seat, we in St-Aubin knew what was going on with CGA-157419 in Basle. Among other reasons because we took part in the yearly New Product Portfolio review, where progress of all research and development projects were presented to the management and intensively discussed. The NPT of CGA-157419 came soon to the conclusion that the most promising approach for such a systemic product for cattle was to develop an injectable formulation, and all work focused on such a formulation. In those years, all cattle tickicides were for topical administration, either as ready-to-use pour-ons or as concentrates for dipping and spraying. The 1% ivermectin injectable didn't (and still doesn't) offer sufficient tick control on cattle, although our Merial competitors worked hard to push it in this direction in Latin America. An injectable tick preventative with a claim of 12 weeks protection should be positioned as a “vaccine-like” product, with numerous competitive advantages towards classic dips, sprays and even pour-ons, which dominated the tick market in those years. Such a “vaccine-like” product would be really new, unique, different and superior to any other tickicide then in the market: just the best-case scenario according to all marketing handbooks.
 However, several initially promising uses (e.g. against fowl mites, scab and mange mites, etc.) had to be abandoned along the development project. Either efficacy was insufficient (e.g. against mange and scab mites), or residues too high. In fact CGA-157419 is a very lipophilic molecule and residues in eggs were unacceptable for a poultry parasiticide. The high milk residues forced to give up the dairy cow market as well. What was left was a tickicide for beef cattle, mainly in Australia, South America, and Southern Africa, the countries were cattle ticks, Rhipicephalus (Boophilus) microplus or R. decoloratus were and remain a major problem.
However, several initially promising uses (e.g. against fowl mites, scab and mange mites, etc.) had to be abandoned along the development project. Either efficacy was insufficient (e.g. against mange and scab mites), or residues too high. In fact CGA-157419 is a very lipophilic molecule and residues in eggs were unacceptable for a poultry parasiticide. The high milk residues forced to give up the dairy cow market as well. What was left was a tickicide for beef cattle, mainly in Australia, South America, and Southern Africa, the countries were cattle ticks, Rhipicephalus (Boophilus) microplus or R. decoloratus were and remain a major problem.
With its market potential shrinking, the whole project shifted from very promising, to simply promising. However, a shrinking market potential also meant a shrinking interest in the management, and consequently a lower priority when competing for resources with other development projects (e.g. milbemycin oxime to become INTERCEPTOR, and lufenuron to become PROGRAM). As I previously mentioned, the highest priority for new products was to target the “TRIAD”, meaning US + Europe + Japan. You didn’t need to be very smart to anticipate that CGA-157419 injectable for beef cattle against tropical ticks could run into trouble: there was no significant market for it in the TRIAD.
Another threat came from the development costs. In the past most new ectoparasiticidal active ingredients in Ciba-Geigy had been developed and used together by Crop Protection and Animal Health. In fact, Animal Health was and remained a subdivision of Ciba-Geigy’s Crop Protection Division until 1996, when Ciba-Geigy and Sandoz merged. The common joint development of active ingredients meant sharing the development costs, particularly those of the toxicology package and of process development for manufacturing. In those years these two packages made more than 50% of the total development costs. Crop Protection having no interest in CGA-157419, AH had to finance all these costs alone, in those years about $10 million, not precisely peanuts.
But things worsen when it was found out that the injectable formulation left prohibitive residues at the injection site. All attempts to solve this problem by improving the formulation failed and delayed the project by years. In a last effort to save the project, the NPT decided to try a pour-on formulation, which wasn’t easy either, because CGA-157419 is virtually insoluble in most solvents accepted by the usual pharmacopoeias. Fortunately they were able to quickly produce a viable pour-on formulation and to proof in a record time that it worked as well, or even better than the injectable. But about 4 yeas were lost trying to get the injectable through. In the meantime both CGA-157419 and the new product had got their own names: fluazuron and ACATAK, respectively.
April 1991. Joining the fray
In the meantime it was April 1991. It came what frequently happens in many companies: another re-organization. Since April 1985 I had "survived" two major and several minor re-organisations. The last one had even meant a promotion for me and I was now head of the whole in-vitro and pre-clinical endo- and ectoparasiticidal screeening. This new re-organisation affected the whole AH Research Center in St-Aubin and was caused by the shut down of the BioVet Unit: all the projects they had been working on for about 6 years had failed to achieve the expectations. But in those days Ciba-Geigy was still a typical Swiss-styled, conservative company: there was an amazing strong mutual loyalty between company and employees, nowadays completely unknown. Hire & fire was not an option and still politically incorrect: it became common practice only shortly after the merger of Ciba-Geigy with Sandoz to create Novartis in 1996. In 1991 Nodody was fired except in manifest but unusual cases of fraud or incompetence. And very few people left the company by their own initiative.
 In 1991 the BioVet team was not fired (about a dozen out of a group of 60 employees in the whole Research Centre). It was not guilty of not meeting the expectations of the Management. It had just shown that the expectations on the new biotechnologic molecules were irrealistic. This was aknowledged by the Management, at least tacitly. And nobody in the Management involved in the previous creation of the BioVet Unit based on irrealistic expectations was fired either. It was probably accepted that "no risk - no business". And the whole Research Department was re-organized.
In 1991 the BioVet team was not fired (about a dozen out of a group of 60 employees in the whole Research Centre). It was not guilty of not meeting the expectations of the Management. It had just shown that the expectations on the new biotechnologic molecules were irrealistic. This was aknowledged by the Management, at least tacitly. And nobody in the Management involved in the previous creation of the BioVet Unit based on irrealistic expectations was fired either. It was probably accepted that "no risk - no business". And the whole Research Department was re-organized.
I was not in the BioVet Unit. But there were obviously too many people in the Research Station. My boss had asked me whether I would agree to move to Basle. I have answered that I wasn't keen at all on a transfer to Basle, but would do it if there was no alternative. After months of rumors and speculations a meeting was called around mid March 1991 to communicate the new organization in the research center. I was not invited: obviously I was not going to be part of it. But my boss had told me that I would get a phone call from Basel.
Indeed, I got that phone call a few hours after that meeting, still the same day. It was my future boss in Basle that offered me a job: Product Manager for Livestock Ectoparasiticides with worldwide responsibility. This meant leaving research and going to marketing, something I had never considered before. I asked for a few days to think it over, because I had no idea about marketing, which I specifically said to my future boss. He answered that I would get adequate training for this.
It was quite easy to decide. Basically because I didn’t have an alternative, since somebody else had already got my previous job (my former boss: from now onwards he was supposed to do himself what we had being doing both together). And the new job seemed not unattractive. Regarding my total marketing ignorance, most scientists in those years, including ourselves in St-Aubin, joked about marketing telling something like “the only important thing to know about marketing is that there is nothing important to know about marketing”. I must admit that I rather agreed. I was soon to learn that I was wrong.
I accepted the job in Basle. We were a group of four to leave the CRA for Basle: two from the BioVet unit and to from the Screening unit: very equitable, very Swiss.
It happened that my predecessor in Basle was precisely the responsible for marketing within the New Product Team for fluazuron. So with the job he handed me also this project. After years watching fluazuron from the fence, I suddenly joined the fray.
This was April 1991. And the yearly Portfolio Review was scheduled for a few months later. Fluazuron’s progress and perspectives had to be presented to the management. My new boss set me the target: keep the project alive. To make it more exciting, my NPT colleague that was really familiar with the project was not in Basle but in Australia, where he had move months before, just to push the fluazuron project there. The reason was that much of the development work was being done in Ciba-Geigy’s research station down-under (in Kemps Creek, close to Sydney) and Australia was also the key target market. So I had to defend the fluazuron project completely alone. I was familiar enough with ticks and fluazuron to defend the technical aspects, but I had never been in Latin America, and only a few days in Australia and South Africa, i.e. I had now idea about the market.
Marketing: Facts or perceptions?
As a scientist, I was used to deal and argue with data, facts and evidence (derived from experimental results, calculations, etc.), and to draw the rather obvious conclusions that any other colleague would agree upon based on the same data. So I collected data, analyzed results, calculated scenarios, etc, and put a lot of “facts” into a few acetates (the presentation standard before PowerPoint). Not too many acetates (I had only 15 minutes for my presentation) but crowded with figures and calculations. I hoped that all those facts and figures would proof the management the evidence that it was worth continuing the project.
After having gone through my acetates, the big boss just asked one question: “Can you make a successful product out of it?” Feeling the sudden adrenaline shot I thought: Pablo, no more data; yes or no. This is all he wants to know and this is your last chance. And I said, 18 years before somebody else: “Yes we can”! And the fluazuron project survived. It was not a matter of facts and data (most managers knew very little about ticks, pour-ons, systemic effect, etc.): what was decisive was how they perceived me selling the project: if the guy is convinced, let him do it… Obviously managers are rather normal people.
And this was the first important thing I learned about marketing: it’s not about facts, but about perceptions. This means that data and facts about a commercial product are not decissive, but how people perceive it. You cannot influence data and facts. But you can influence perceptions...
An off-budget marketing crash course
 Still believing that there were not many important things to learn about marketing, I quickly found out that marketing people had an own slang. I felt often offside when talking to my new colleagues: they used terms that I understood, but I didn’t know what they were meaning. In any case, my boring tech slang needed an upgrade. I found out that the Training Department of Ciba-Geigy’s Agro Division offered marketing courses for beginners and proposed my new boss to join the next one in September: one week in a small Seminar-Hotel in Thun, a charming small town very close to Interlaken in the Swiss Alps.
Still believing that there were not many important things to learn about marketing, I quickly found out that marketing people had an own slang. I felt often offside when talking to my new colleagues: they used terms that I understood, but I didn’t know what they were meaning. In any case, my boring tech slang needed an upgrade. I found out that the Training Department of Ciba-Geigy’s Agro Division offered marketing courses for beginners and proposed my new boss to join the next one in September: one week in a small Seminar-Hotel in Thun, a charming small town very close to Interlaken in the Swiss Alps.
To my surprise, my new boss was not very enthusiastic about my hunger for knowledge and said: “... but it’s not in the budget.” This was a first hint on the different approach to budgets in the marketing and the research departments. I insisted and remembered him his first phone call promissing adequate training. He gave up and I joined that marketing course in Thun, where the mysteries behind SWOT-Analysis, Competitive Advantage, Positioning, Differentiation, Segmentation and other crucial marketing secrets were unveiled to me. And after that course I didn’t feel any more as a foreign body in the marketing-driven ecosystem of Ciba-Geigy AH headquarters in Basle.
Full-speed ahead in Australia
Soon after, my development colleague in the NPT returned from Australia but changed the job, and a new guy joined the NPT: Hariolf Schmid, who was to become the most reliable and straightforward colleague I ever had. I was to share with him quite some unexpected challenges while struggling to bring fluazuron (and other projects) to market. Each NPT was strongly supported by a Registration Manager and a Formulation Manager, for ACATAK Gerhard Hool and Walter Oechslein, respectively.
The field trials in Australia using the new pour-on formulation confirmed all our expectations and about 2 years later the dossier was submitted for registration in Australia. For registration there, the major challenge was not efficacy, but residues: the MRL (Maximum Residue Limit) in cattle tissues. The MRL accepted by the authorities determines the withholding period, i.e. how long a farmer has to wait between the last treatment and slaughtering for human consumption. Ideally the withholding period is nil, i.e. 0 days. A long withholding period is obviously a disadvantage, particularly if competitors have a shorter one. In those days, MRL’s for most common cattle parasiticides ranged from 0.1 to 1 ppm. Longest withholding periods were about 4 weeks.
Once absorbed into blood fluazuron is deposited in the animal’s fat where it builds a reservoir. It is not metabolized in the fat but slowly released back into the blood. This allows keeping the fluazuron concentration in blood that is effective against ticks during 12 weeks and beyond. But this also means that residues in fat tissues remain quite high for weeks. Based on the very low toxicity of fluazuron to mammals and following the usual criteria for determining the basic parameters (NOEL = No Observable Effect Level, ADI, acceptable daily intake, etc.) we concluded that an MRL of 10 ppm in fat and above would be technically justifiable. But we also knew that such a high MRL was not politically correct in Australia and elsewhere, already in those days. We decided to go for an MRL of 7 ppm in fat, still very ambitious, which would result in a withholding period of 6 weeks, still acceptable for us.
In addition to the withholding period for local consumption, Export Slaughter Intervals (ESI) were required in Australia for active ingredients that were not registered or had no import tolerance in target export countries. Such ESIs ensured that when cattle were slaughtered for export, the residues of such active ingredients had dropped below the detectable level. For ACATAK an ESI of 12 weeks had to be expected. It was not prohibitive because ACATAK had the claim of preventing cattle tick infestations for up to 12 weeks: we assumed that most farmers would not use ACATAK on cattle scheduled for slaughtering earlier than this.
After submitting the registration package in Australia there was not too much else to do for us in Basel but to wait and see what happened.
Bad news from Latin America
Soon after moving to Basle I started to travel regularly to Latin America, not only to explore the market for ACATAK, but also to support the local teams in anything related with livestock ectoparasiticides. Regarding ACATAK, the key countries were obviously Brazil, Argentina, Colombia and Mexico, based mainly on their large cattle populations, but also on our local resources there in those years.
Due to the previously mentioned problems with the injectable formulation, the pressure for pushing the pour-on project in Australia, and the change in the NPT, the field trials with the pour-on formulation in Latin America started only after ACATAK had been submitted for registration in Australia, or shortly before.
Fieldwork started in Brazil, where we had identified the largest potential and where we had a good local support. The first results were very disappointing. We didn’t get 12 weeks control, but something between 6 to 8 weeks. We found the explanation quite rapidly, reviewing all the data we had from previous work. Early in-vitro tests with fluazuron against the South American R. microplus strain MOZO had shown that these ticks were less susceptible to fluazuron than the Australian strains (YEERONGPILLY, BIARRA, PARKHURST, etc) we reared in the laboratory, i.e. they needed a higher dose than the Australian ticks to be controlled.
Based on this information we concluded that the 1.5 mg/kg dose used in Australia was just too low against Latin American R. microplus ticks and we decided to try a dose of 2.5 mg/kg. Obviously without changing the formulation, which would have been a nightmare. A new set of field trials at this dose yielded excellent results, comparable to those in Australia.
But, we lost a lot of time. And worse: a higher dose meant that the margin would drop, because we could not raise the end-user prize accordingly, since it was imposed by the competitive situation. This was an additional internal hurdle for ACATAK within Ciba-Geigy, because we were still competing for internal resources with other large projects promising very high margins in the pet market.
 After several trips to Brazil, Colombia, Mexico, Venezuela, Guatemala and other Latin American countries we had to further reduce our expectations regarding market potentials. The more countries I visited, the more I discovered that most beef cattle in Latin America from Mexico down to Brazil were either pure Zebu (i.e. Bos indicus) or crossbred (i.e. Bos indicus x Bos taurus) cattle. Pure European breeds were used almost exclusively for milk production in those countries. The predominance of pure Zebu or crossbred cattle had serious implications for ACATAK’s market potential. We knew already that pure Zebu cattle are much less susceptible to ticks than crossbred or European cattle. And in fact, in most places in those countries such cattle were not at all or only occasionally treated against ticks. Their major ectoparasiticidal problem in Zebu cattle in those years was horn flies (Haematobia irritans), not cattle ticks.
After several trips to Brazil, Colombia, Mexico, Venezuela, Guatemala and other Latin American countries we had to further reduce our expectations regarding market potentials. The more countries I visited, the more I discovered that most beef cattle in Latin America from Mexico down to Brazil were either pure Zebu (i.e. Bos indicus) or crossbred (i.e. Bos indicus x Bos taurus) cattle. Pure European breeds were used almost exclusively for milk production in those countries. The predominance of pure Zebu or crossbred cattle had serious implications for ACATAK’s market potential. We knew already that pure Zebu cattle are much less susceptible to ticks than crossbred or European cattle. And in fact, in most places in those countries such cattle were not at all or only occasionally treated against ticks. Their major ectoparasiticidal problem in Zebu cattle in those years was horn flies (Haematobia irritans), not cattle ticks.
Only in Argentina and Uruguay European breeds dominated the beef cattle industry. But in those years, these two countries ran a tick eradication campaign, which meant that large regions were already tick-free (i.e. no market for a tickicide), and requirements for product registration were very strict and rigid. ACATAK didn’t fit in the registration requirements of Argentina because it had no knockdown effect. All this meant that the potential market for ACATAK there was rather modest and we didn’t know yet how to get the product approved.
One of the major ACATAK benefits was (and remains) that it works against any kind of resistant ticks. Pyrethroids dominated the tickicide market in the 1990s, market leaders being BAYTICOL (flumethrin) and BUTOX (deltamethrin). Organophosphates had been vastly abandoned in Latin America in those years, superseded by pyrethroids that were as effective but less toxic. Amitraz had never been very popular, because in contrast with pyrethroids (and organophosphates) it doesn't control biting flies and is unstable in dip vats. And IVOMEC (the original 1% ivermectin injectable), which was the Rolls Royce among parasiticides, was not effective enough for tick control. The same applied to other injectable macrocyclic lactones (moxidectin, doramectin, etc.) that had just entered the market.
Tick resistance to pyrethroids was an opportunity for ACATAK, and we expected that it would spread and increase quickly. But traveling around I found out that from Brazil to Mexico, under tropical and subtropical climates, the most serious problems with tick resistance to pyrethroids (and/or organophosphates) occurred in dairy farms, the vast majority of which were grass-based farms. The reason was that the overwhelming majority of dairy cows were of European breeds (Holstein, Swiss Brown, etc.) intensively treated with pyrethroids, frequently every 3-4 weeks the whole year through, to protect them from both ticks and biting flies. And as I previously mentioned ACATAK was unsuitable for dairy cows.
I also discovered, that between Peru and Mexico, a significant part of small to mid-sized cattle farms (i.e. up to ~2’000 heads) are so-called dual-purpose, i.e., beef and dairy. And in smaller farms (up to ~300) another practice was also very common with dairy cows: two tits for the calves and two tits for the workers. In fact, when visiting many countries, my local colleagues took me very often to visit such dairy or dual-purpose farms, because they knew that they were highly bothered by resistance problems and would easily change to ACATAK.
You can imagine what this meant regarding ACATAK’s market potential and marketing there, if it was not to be used on cows producing milk for human consumption.
I put my NPT colleague Hariolf under considerable pressure to find a solution that would allow using ACATAK in dairy cows producing milk for human consumption. He tried hard but his calculations clearly indicated that there was a significant risk that the mother’s milk of nursing women regularly drinking milk from fluazuron-treated cows would contain detectable fluazuron residues, and that such residues could even be detectable in suckling babies as well. And that was it. I surrendered.
About "bad" ticks and "worse" ticks
My numerous trips to Latin America also confirmed what we already knew regarding the tick species prevailing in cattle. The one-host-tick R. microplus is the predominant and almost only relevant tick species for livestock in most of Brazil, Argentina, Uruguay, and central parts of Mexico. We can call them "bad" ticks because they bite one (and only one) animal during their life cycle.
 In contrast with this, the three-host tick Amblyomma cajennense bites three different hosts during its life cycle, the reason for considering it a "worse" tick. It often occurs together with R. microplus and can become the predominant species, particularly in tropical regions in the Caribbean, from Mexico to Venezuela, as well as in tropical Brazil. Whereas R. microplus infects mainly cattle and deer, A. cajennense infects all kinds of mammals: cattle, sheep, goats, horses, dogs, cats, humans, etc.
In contrast with this, the three-host tick Amblyomma cajennense bites three different hosts during its life cycle, the reason for considering it a "worse" tick. It often occurs together with R. microplus and can become the predominant species, particularly in tropical regions in the Caribbean, from Mexico to Venezuela, as well as in tropical Brazil. Whereas R. microplus infects mainly cattle and deer, A. cajennense infects all kinds of mammals: cattle, sheep, goats, horses, dogs, cats, humans, etc.
ACATAK works very well against R. microplus, but not that well against A. cajennense and other nasty two- and three-host ticks. To understand why I have to go into some biological details on tick biology and farmers' psychology.
For R. microplus ticks, only larvae are free-living on pastures and infect cattle. But larvae are too small to be perceived by the farmers. Consequently, cattle infected with thousands of R. microplus larvae are perceived by farmers as tick-free, simply because they don’t see them. In untreated cattle, these thousands of larvae will feed blood and molt to nymphs (still to small to be perceived), without leaving the host. Nymphs also feed blood and molt to adults without leaving the host. Once adult females suck blood, they swell up to the size of a bean and become visible for farmers, about 3 weeks after larvae infected the animals. This means that rather suddenly a supposed “tick-free” animal becomes infected with hundreds or even thousands of bean-like engorged adult ticks. On cattle treated with ACATAK, most larvae will die during their first molt to nymphs. As a consequence farmers won’t see bean-like ticks on their animals and that’s what they expect to happen after treating their cattle with ACATAK.
In contrast with this, larvae, nymphs and adults of A. cajennense are free living on pastures, and will infect cattle grazing there. On untreated cattle, all will bite, attach, suck blood, detach and drop to the ground. In the ground, larvae will molt to nymphs that will infect new animals, nymphs will molt to adults that will infect new animals, and adult females will lay eggs (and die), which produce larvae that will infect new animals. On cattle treated with ACATAK, larvae of these ticks will feed blood, drop to the ground and die there during molting, whereby farmers don’t see the larvae because they are too small. The same will happen with nymphs and adult ticks that infect cattle treated with ACATAK. But after engorging and before they detach and fall to the ground, they reach a size that is already visible by farmers, particularly the adults, which are larger than R. microplus and can become as big as an olive. After detaching, nymphs will die during molting, and eggs of adult female ticks won’t hatch, which strongly decimates the tick populations in the pastures. But the bottom line for a farmer is that he sees large nasty ticks on the animals he treated with ACATAK, something he doesn’t like at all.
This meant that ACATAK was not appropriate for regions with high incidence of A. cajennense ticks. Later on we run several trials that showed that applied strategically ACATAK gave excellent results in reducing A. cajennense populations in Mexico. The reason is that although all free-living stages can be found on pastures the whole year through, larvae predominate in early spring, nymphs in late spring and early summer, and adults during the summer. Starting ACATAK strategic treatments in early spring made it possible to massively reduced the A. cajennense tick populations on pastures. But it required to convince farmers to treat their animals months before they were used to do it with conventional tickicides based on their main criteria for treatment: seeing “visible” ticks on their animals.
For the same reason we anticipated little potential for ACATAK in Africa, because two- and three-host “worse” ticks are rather predominant in most tropical and subtropical regions there, including Kenya and South Africa, the largest African tickicide markets in those years.
1994. Under the volcano in Australia
We finally got the market authorization for the ACATAK pour-on in Australia in 1994. This was a record time, considering, that development of this pour-on formulation had started in 1991. After years of efforts to eradicate R. microplus in Australia, large parts of the country were already tick-free. Queensland was not, and all our marketing efforts concentrated there.
In those years, most beef produced in Australia was for export, mainly to the US and Japan, but also to Canada, Europe and Korea. Since fluazuron was to be used only against cattle ticks, ACATAK and any other product containing fluazuron would never be registered in these export destinations, simply because there are no Rhipicephalus (Boophilus) microplus ticks there. We were very much aware that fluazuron residues in export beef could become an issue. To tackle the potential residue issues in export beef we had planned to apply for CODEX registration of fluazuron as soon as possible. The Codex Alimentarius Commission (usually called CODEX) was established by FAO and WHO in 1963 to harmonize international food standards, guidelines and codes of practice to protect the health of the consumers and ensure fair practices in the food trade.
However, Ciba-Geigy could not submit a dossier to CODEX directly. This had to be done by FAO member countries were the product had been already registered. After submission, it would take about 2 years for a CODEX-MRL to be approved. In the meantime ACATAK had also been submitted for registration in Brazil, and we had planned to ask the registration authorities in Australia and Brazil to submit fluazuron to CODEX as soon as possible.
Nevertheless, in those years large member countries (e.g. USA, EU, CANADA, JAPAN) had not committed to accept CODEX MRLs, although they would usually indulge them. It also happened in these countries that their own MRLs for many chemicals were often different from those of other countries or CODEX. We knew e.g. that the US didn’t make an issue with cyromazine residues in mutton imported from Australia. Cyromazine was registered in the US but only for use on poultry, not on sheep. However, mutton was not a big business in the US. Flumethrin and amitraz, two other tickicides vastly used in tick countries, including Australia, were not registered in the US for use on cattle, and there had been no issues with illegal residues so far.
Thus, we were confident that this potential issue would be manageable, considering also that being a tick development inhibitor and not a “tick killer” fluazuron was extremely safe for humans.
Later on in 2002 Bayer voluntarily withdraw its flumethrin pour-on BAYTICOL from the Australian market precisely because trade trouble with the USA was expected to occur.
Remember Murphy: Anything that can't go wrong, will go wrong.
Very soon after the launch of ACATAK in Australia in 1994 we learned that a big issue with illegal residues in thousands of beef cattle in Queensland was going to burst. The Australian authorities had just found excessive residues in beef that they couldn’t identify. Nobody knew yet what it was about. It couldn’t be fluazuron, because it was only a few months after the launch.
The authorities finally found out that the illegal residues were chlorfluazuron, another benzoylphenyl urea, closely related to fluazuron, but not approved for use on cattle in Australia or anywhere else in the world yet. They immediately informed the US, Japan and other beef importing countries, which immediately banned Australian beef imports until the issue was cleared. And than the s… hit the fan, with breaking news in the Australian media. There were thousands of cattle contaminated with chlorfluazuron that the farmers could not slaughter for export.
What had happened? How did chlorfluazuron end in those cattle? There had been a severe drought in Queensland that year. And many Queensland farmers had fed cotton trash to cattle, because pastures had dried out. But much of that cotton had been treated against cotton parasites with chlorfluazuron, recently approved for that use in Australia. Being a highly lipophilic molecule, chlorfluazuron ingested by cattle is deposited in the fat tissues where it tends to accumulate because it is hardly metabolized and only slowly released and excreted. It seems that the Australian authorities were able to identify the residues as coming from chlorfluazuron thanks to the analytical method for measuring fluazuron in beef samples delivered by us with the ACATAK registration dossier: fluazuron and chlorfluazuron are chemically very close.
This was in fact a worst-case scenario for ACATAK. Chlorfluazuron sounded suspiciously close to fluazuron. Arguing that fluazuron was approved for use on cattle and completely different from chlorfluazuron would convince nobody. And fluazuron was neither registered nor had an import tolerance in Australia's major beef export countries. And under such a pressure from the public opinion we couldn’t expect indulgence in the US or elsewhere regarding potential unapproved fluazuron residues that may be detected in imported beef from Australia.
In cooperation with the Australian authorities our local management decided to voluntarily withdraw ACATAK from the Australian market to wait for the storm to calm down.
Shortly afterwards the Australian registration officials signaled us that re-introduction of ACATAK would only be possible after getting approved import tolerances in the major countries that imported Australian beef: namely the US, the EU, Canada, Japan and Korea. If I recall correctly, all but one of these countries had already established procedures for applying for import tolerances for veterinary products. All but the US, which was an absolute must, because it was the largest export market for Australian beef.
In fact, we had already planned to apply for import tolerances in key beef-importing countries, but had not yet started to work on it, naively assuming that things that can go wrong wouldn’t necessarily go wrong everywhere, suddenly and altogether.
A Gordian knot
The problem in the US was a Gordian knot. The US EPA had a procedure for approving import tolerances for pesticides not registered in the US. But not the FDA, which was the responsible authority for veterinary medicines. And it was not clear, whether ACATAK fall under the EPA or the FDA jurisdiction. EPA was responsible for livestock ectoparasiticides, and ACATAK was a livestock ectoparasiticide. But FDA was responsible for active ingredients with a systemic mode of action, and fluazuron has a systemic mode of action. After several inquiries and requests it was concluded that we had to knock at the FDA’s door.
The point was that the FDA didn't have an established procedure for approving import tolerances. Consequently we didn’t know how to apply, which documents we had to deliver, which studies we had to run, etc.
Davids against Goliaths
You may think that Ciba-Geigy was already a big multinational company, with plenty of money and other resources, capable of putting pressure on the FDA to take action. If you do, you are wrong. In fact, Hariolf and myself we rather felt like Davids facing several Goliaths.
The first Goliath was Ciba-Geigy’s own local Animal Health team in the USA. In the USA in those years, Ciba-Geigy Animal Health had limited resources and no livestock business at all. The small development and registration team was fully busy with several pet projects (lufenuron, milbemycin oxime, etc.) that had highest priority for them as well as for the Basle headquarters, because they had a very high market potential, much higher than the one of ACATAK worldwide. And ACATAK had no potential in the US because R. microplus ticks were eradicated long ago. Now, two guys, a German and a Spaniard show up there and asked for resources to be put into a project that had zero market potential in the USA. And they wanted to get something that didn’t exist, an FDA import tolerance. Guess how enthusiastic they were about it. But in contrast with the Bible’s Goliath, they helped us, mainly by bringing us in contact with several consultants that could help. We eventually selected one. Not a development or registration specialist, but a lawyer. The other Goliath was the FDA.
Asking Bill Clinton for help
We took a lawyer because we came to the conclusion that the only realistic approach to get a solution was to file a so-called “Citizens Petition” to the Government of the US asking that the FDA establishes a procedure for setting import tolerances for veterinary medicines not approved in the US. You probably understand why Hariolf and myself we felt like David facing a further Goliath when considering this approach.
 I was not very optimistic that this would work. And if ever, I rather feared it would take 4 to 6 years to happen, too long for our management to wait. But there was no real alternative.
I was not very optimistic that this would work. And if ever, I rather feared it would take 4 to 6 years to happen, too long for our management to wait. But there was no real alternative.
If someone in Ciba-Geigy AH could do it, it was Hariolf. And he took the challenge. And believe it or not, after numberless, e-mails, calls, reports and meetings, in about two years our citizens’ petition was accepted! One day Hariolf received a copy of the original document signed by Bill Clinton! He framed it and hanged it beside his office door. And he and me, the New Product Team for ACATAK, we got a (rather modest…) bonus that our boss had promised us if we get the problem solved. We both got a bonus, but it was 100% Hariolf’s job. Unfortunately, Bill Clinton’s framed signature went lost in a later re-organization…
The approved citizen’s petition basically asked the FDA to set up an application procedure for approving import tolerances. But such a procedure had to be worked out and approved, something that usually takes years of internal discussions, meetings, etc. We didn’t feel at all capable of putting any pressure on mighty FDA to accelerate the process, and feared that it would take years, because the FDA had for sure more important things to do. And once a procedure was established, would we need to run more studies to supply data we didn’t have? If yes, running such trials could take more years!
While waiting we concentrated our efforts in Latin America.
A completely new approach to tick control
Fluazuron was the first tick development inhibitor in the tickicide market. More than twenty years later it still remains the only one. It was definitely new, unique and different. It was and remains superior, but only if correctly used, which can be tricky. And it was and remains different, very different. Too different perhaps, at least in those years.
Its major drawback was and remains the lack of knockdown effect on ticks. Until then, all tickicides in the market were basically tick killers. This meant that regardless of the type of cattle they were used on (age, breed, beef or dairy, etc.) the time of the year and the tick species, they would quickly kill the bean-sized engorged ticks attached to cattle. Something most farmers could see with their own eyes within a few hours after treatment.
In most places, R. microplus tick infestations follow a seasonal pattern. Ticks start to be a problem in early to mid spring, peak in mid to late summer and spontaneously disappear (from cattle) during winter. Depending on the climatic conditions 3 to 5 generations can follow during a season. What farmers see are the attached engorged adult female ticks, and most of them were used to dip or spray their cattle when they felt (i.e. they saw) that they carried "too many" engorged ticks. All tickicides would actually kill most of those “visible ticks” and after treatment cattle would look tick-clean for several weeks.
I have already explained the basics of the R. microplus life cycle, which determines how ACATAK has to be used to be successful, and how ACATAK breaks this cycle by blocking the molt process of larvae to nymphs and of nymphs to adults (they die when attempting to molt), and by inhibiting hatching of eggs deposited by engorged adult females once thy drop to the ground.
If ACATAK is applied to cattle already infested with engorged female ticks, it won’t kill any of them. It will kill only the microscopic larvae and nymphs when they attempt molting. This means that treated animals will be tick free only when the last engorged females naturally drop. The benefit is, that the eggs of those engorged females will not hatch, and that once they have dropped no more adult ticks will develop out of the larvae and nymphs that were there, because they have died when attempting to molt. But it takes 2 to 3 weeks for tick-infested cattle to become tick free after treatment with ACATAK. This requires a lot of patience from farmers that have paid an expensive price for ACATAK.
Therefore ACATAK has to be used strategically, i.e. not when cattle are already infested with visible engorged adult female ticks, but before. This meant that we had to convince farmers to treat their cattle with ACATAK (a pretty expensive product) already in early spring, much earlier than what they were used to, i.e. when they still perceived no ticks on their animals. If you think that changing the farmer’s mind wouldn’t be too difficult, you don’t know farmers, at least not Latin American cattle farmers.
For these reasons it was clear for us that ACATAK marketing could not be done as usual, i.e. pushing it into the shelves in as many agro-shops as possible, and making a lot of noise and advertising. The risk that untrained farmers used it incorrectly and were disappointed was enormous, which would very quickly kill the product.
ACATAK was not only new and different for farmers, it was also new and different for distributors and for our own technical and sales people in the various countries.
Since ACATAK was also quite expensive, we concluded that initial marketing should focus on large innovative farmers that should be identified, motivated and trained accordingly. And the trainers had to be trained as well. This strategy required significant investments in people and resources for the new product. Among other reasons because the sales force dealing with livestock parasiticides was quite weak in several key countries. And it also needed time.
I soon realized that successful marketing of ACATAK also required convincing the local management that ACATAK was a good product for them, that this strategy was adequate, and that the required investment would pay back. To this aim I visited the key countries rather frequently, we held two regional workshops for country Product and Sales Managers in Londrina (Brazil, 1995) and Villahermosa (Mexico, 1996), and we started countless local field trials to show our people and key customers that ACATAK really delivered.
Precisely this task of training our people proofed to be particularly fragile, because people changed jobs “too quickly” for reasons that had little or nothing to do with ACATAK. And then all the energies invested in training and convincing those people were lost. In Mexico, one of the key countries for ACATAK, the Country Manager changed and both the Product Manager responsible for livestock parasiticides and the Sales Manager left the company shortly before or after the launch of ACATAK. The same happened in Australia, another key country, with both the Development Manager and the Product Manager.
Thinking small and and acting slowly?
The top management in Basle had declared pets as the first priority worldwide, including Latin America. The reason was that in the meantime PROGRAM (the first monthly pill against fleas based on lufenuron, another development inhibitor) had experienced a tremendous success in the USA that had to be replicated worldwide, including Latin America. The slogan for PROGRAM was "Think big". And the objective was ro reach plateau sales asap, not later than 3 years after the launch.
The management in Latin America was not convinced that such an expensive pet product like PROGRAM was the best for their markets strongly driven by livestock, where pet products addressed only a small affluent minority in the large cities. Time would show that they were right, but they had no alternative but trying. As a consequence, although most of them were convinced that ACATAK was very promising for their country, they could not allocate all the resources that would have been required for pushing ACATAK stronger and faster.
The strategy we followed with ACATAK avoided publicity and did not target fast growth, i.e. we accepted that it would not become a bestseller soon, even less a fast-seller. But we wanted it to be at least a long-seller. Unfortunately, this strategy was clearly in contrast with the management’s global strategy for PROGRAM, which consisted in starting everywhere with a huge advertising campaign, and maintaining the advertising pressure in order to achieve plateau sales within 3 to 4 years. All under the mentioned official global slogan: THINK BIG! Well, we were obviously not thinking big enough with ACATAK.
It is useful to know, that PROGRAM had become the first “consumer” product of Ciba-Geigy, and that the huge advertising campaign in the USA (replicated later in many other countries) was the first such campaign at all run by an Animal Health company for a new veterinary medicine. About 20 million US$ were put into that first campaign alone in the US. Success was overwhelming and PROGRAM sales rocketed in the US. This strategy was a big success in many “rich” countries. But it didn’t work in most Latin American countries, at least not to meet the expectations of the headquarters.
The trouble for ACATAK was that inside the company we were swimming against the tide: We were thinking small and progressing slowly.
1996. Instantly fired – instantly hired
During one of my trips, in December 1996, I got a phone call from Hariolf while staying in Bogotá. His message was short and precise: “You don’t work anymore for Ciba-Geigy”. After a few seconds, enough for a sudden adrenaline shot, he added: “You now work for Novartis”. And he updated me on what had been communicated to people in Basle regarding the Ciba-Geigy + Sandoz merger, which had not yet reached Bogotá.
Interestingly, the Ciba-Geigy + Sandoz merger to become Novartis came only months after Ciba-Geigy hade completed a global exercise typical for large companies: changing the corporate image and logo from “Ciba-Geigy” to “Ciba”. This meant that we had just finished changing all our printing materials: letter headings, fonts, brochures, leaflets, logos, labels, visit cards, ads, etc. worldwide. In this respect the merger found us well trained: “play it again, Sam”.
Three days of hell in paradise
Some of you may have read “Thirteen Years of Hell in Paradise”, the book where Rupert Peagram tells his frustrating experience leading the “Caribbean Amblyomma Program”. In those years I heard about that program to eradicate the tropical bont tick, Amblyomma variegatum, from the Caribbean Islands, where it was introduced from Africa in the nineteenth century. Amblyomma variagatum is even nastier than Amblyomma cajennense, among other reasons because it transmits a disease called heartwater, often fatal for cattle.
 I learned that they had planned to use BAYTICOL for it, and I thought that such a program could be an opportunity for ACATAK. It could be also interesting to run some trials with ACATAK there to see if it would control these ticks as well.
I learned that they had planned to use BAYTICOL for it, and I thought that such a program could be an opportunity for ACATAK. It could be also interesting to run some trials with ACATAK there to see if it would control these ticks as well.
In those years I traveled regularly to Cuba and I arranged a detour to meet Rupert at the program’s headquarters in Georgetown, Barbados at the end of January 97. I was staying in a nice Hotel on the beach, which was almost empty because it was not the tourist season. But I expected to have a few hours for relaxing a bit after a hard week in Cuba.
My expectations were terribly disappointed. Guilty was the air conditioning in my hotel’s room. I got a tremendous stiff neck already the first day, and the first thing I asked Rupert was to bring me to a pharmacy to get some relief (VOLTAREN cream, from Novartis…). It helped a bit, but it spoiled my whole stay in Barbados.
We visited some farms and agreed on a trial with ACATAK. Results were not conclusive. I forgot about ACATAK for that program. It was my first and last trip to Barbados.
1998: Leaving the fray
Surprisingly for me, after “winning” the citizen’s petition the FDA didn’t take it easy, but seriously. Only months later they approved a procedure for establishing import tolerances. And we could apply for it with the data we already had, i.e. we didn’t need new studies. We submitted the requested data package and a few months later we finally got the import tolerance for fluazuron approved, precisely an MRL of 7 ppm in fat, the same as in Australia. In the meantime we (i.e. Hariolf…) had already got the import tolerance of 7 ppm approved in the EU, Canada, Japan and Korea, removing all the hurdles for the relaunch in Australia.
The import tolerance for fluazuron in the USA was the first such import tolerance at all approved by the FDA for a veterinary medicine and in fact opened the door to the approval of import tolerances for other active ingredients not approved in the US but used in other countries that exported beef or other animal commodities to the USA. During the whole process, which was public, we were approached by other large AH companies facing similar problems, offering support, requesting information, etc.
The Australian registration authorities were enthusiastic and very grateful towards Novartis, because the approval of import tolerances by the FDA had been a yearlong objective for them that was finally achieved by those mates from Novartis. It should make possible to eliminate several Damocles swords swinging over Australian beef exports due to livestock medicines approved in Australia but not in the USA or elsewhere.
After getting the import tolerance approved in the US, we started to prepare for the re-launch in Australia. But in the meantime, for reasons unrelated with ACATAK, I had decided to leave Novartis to take the challenge of becoming an independent consultant, which became effective in June 1998.
So I left the fray joined seven years before. One of the most painful consequences of leaving the fray was to interrupt the contact and cooperation with so many excellent colleagues and friends worldwide. I remained in close contact with some of them, particularly with Hariolf and other colleagues in Basle, but not with most of those in Latin America and Australia. I want to express here my gratitude to all of them, among which I particularly remember:
- Carlos Hereu and Pablo Vazzoler, in Argentina
- Frazer Bowen, Tom Friedel, David Overend, Michael Strong, Shane Swindale, Geoff Williams and Rod Mitchel, in Australia
- Izone Correa and Carlos Dourado, in Brazil
- Leonardo García, in Colombia
- Cristina Cuarón and David Fernández, in Mexico
- Barry Hyman, in South Africa
- Rafael Etchebarne, in Uruguay
2014
Most Animal Health companies do not publish data on sales of particular products. Consequently I do not know how slow ACATAK sales developed after I left Novartis. I met Hariolf later on several times. I never asked him specifically about ACATAK sales, and he never told me details, perhaps to avoid disappointing me. People tend to spontaneously tell and exult about success stories, and not to talk about failures. Therefore my feeling is that ACATAK sales developed rather slowly and were disappointing during many years.
But recently I intensively searched for fluazuron in the web and was pretty much surprised: I found >20 different tickicide brands containing fluazuron, either alone or in mixtures. They are marketed by local and multinational companies (besides Novartis-Elanco, e.g. Bayer and Zoetis) in Australia, Latin America and South Africa. I have to acknowledge that I was not only surprised but also proud of it.
Before publishing this story (2014) in my website I sent it to Hariolf (still at NOVARTIS, now ELANCO) for comments and corrections. And he told me about the current sales of ACATAK. My spontaneous reaction was ”not too bad”, considering that today there are already numerous competitor brands in key countries.
By the way, no new chemical class of livestock tickicides has been introduced since the launch of ACATAK in 1994. And macrocyclic lactones (eprinomectin, doramectin, ivermectin, moxidectin) as pour-ons and/or long-acting injectables, fipronil, amitraz, and ACATAK (in Latin America also fipronil) have vastly replaced synthetic pyrethroids as livestock tickicides in most countries. But tick resistance to amitraz is now widespread in many regions and first cases of tick resistance to macrocyclic lactones (Brazil, Mexico), fipronil (Brazil, Mexico, Uruguay) and fluazuron (Brazil, Australia) have been already reported. Let's see what comes next.
2017
In 2015 Novartis Animal Health was acquired by ELANCO. Shortly afterwards ELANCO closed the Centre de Recherches Agricoles in St-Aubin but transfered the parasiticides screening to Basle, i.e. the key tool for discovering new active ingredients. In 2017 ELANCO has also definitely closed the screening. This is the rather sad end of more than 50 years tradition and experience in the discovery and development of animal health antiparasitics. Fifty very successful years, because it is there that were dicovered and partially developed 10 antiparasitic active ingredients still used today. Cyromazine and tricabendazole in the 1970s; milbemycine oxime and lufenuron in the 1980s; flazuron, dicyclanil and nitenpyram in the 1990s; monepantel and pyriprole in the 2000s; and lotilaner in the 2010s.
Some of these compounds still dominate their specific markets worldwide, now with dozens of generic formulations: triclabendazole is the leading flukicide; cyromazine and dicyclanil are the leading flystrike preventatives. Other compounds have become standards in their segments, also with dozens of generic formulations: nitempyram for fastest flea control in pets; milbemycin oxime as wormer and heartworm preventative for dogs and cats.
Time will show whether ELANCO is capable of maintaining such an innovation capacity in the future.
NOTICE. I didn't take any notices nor did I keep a diary during my years in Ciba-Geigy and Novartis. The previous pages are just memories that have survived forgetfulness but may not be accurate. They are my very personal current view of those days. Other people involved may have perceived them differently. I apologize for incompleteness or inaccuracy.
If you are interested in "insider" stories like this one on fluazuron, you may wish to visit the articles in this site on the discovery & development of dicyclanil (CLiK) and lufenuron (PROGRAM), two additional IGRs in which I was heavily involved during my years in Novartis AH (1985-1998).