Ivermectin is a broad-spectrum antiparasitic active ingredient used in veterinary and human medicine in dogs, cats, horses ands and livestock against external parasites (lice, mites, etc.) as well as against internal parasites (e.g. roundworms). It is also used against agricultural and household pests. It belongs to the chemical class of the macrocyclic lactones.
Common name: IVERMECTIN
Type: veterinary or human medicine, or pesticide, depending on usage
Chemical class: macrocyclic lactone
CHEMICAL STRUCTURE
EFFICACY AGAINST PARASITES
Type of action: Systemic & contact broad-spectrum ectoparasiticide and endoparasiticide.
Main veterinary parasites controlled: Gastrointestinal and pulmonary roundworms, lice, mites, horn flies; myiasis by screwworms, bot flies, warble flies, etc.
Efficacy against a specific parasite depends on the delivery form and on the dose administered.
Click here for general information on features and characteristics of PARASITICIDES.
DOSING
- Click here to view the article on Ivermectin dose for Dogs
- Click here to view the article on Ivermectin Dose for Cats
- Click here to view the article on Ivermectin dose for Horses, Cattle, Sheep, Goats, Swine, Poultry, etc.
DISCLAIMER: Liability is denied for any possible damage or harm to persons, animals or any other goods that could follow the transmission or use of the information, data or recommendations in this site by any site visitor or third parties.
SAFETY
Oral LD50, rat, acute*: 25 mg/kg
Dermal LD50, rat, acute*: >660 mg/kg
* These values refer to the active ingredient. Toxicity has to be determined for each formulation as well. Formulations are usually significantly less toxic than the active ingredients.
MRL (maximum residue limit) set for animal tissues (e.g. beef, mutton pork or chicken)*:
- CODEX: Yes
- EU: Yes
- USA: Yes
- AUS: Yes
* This information is an indicator of the acceptance of an active ingredient by the most influential regulatory bodies for use on livestock. MRL's for animal tissues may be set also for agricultural pesticides that are not approved for use on animals but are used on commodities fed to animals. A MRL may be also set in the form of an IMPORT TOLERANCE for active ingredients not approved in a particular country but approved for imported animal commodities.
Withholding periods for meat, milk, eggs, etc. depend on delivery form, dose and national regulations. Check the product label in your country.
WARNING: Dogs of some breeds do not tolerate macrocyclic lactones or other medicines (e.g. emodepside) that can cross the blood-brain barrier. They can suffer more or less serious adverse effects if treated at dose rates slightly higher than the recommended ones. Consequently dosing must be as accurate as possible. This is the case for Collies and related breeds, which have a mutation in the MDR-1 gene that affects the blood-brain barrier and makes it more permeable to such compounds than in dogs without this mutation. Besides Collies, other dog breeds have shown similar problems, although the MDR-1 mutation has not been confirmed in all of them. The breeds more affected by this mutation are (% frequency): Collie (70%), Long-haired Whippet (65%), Australian Shepherd (50%, also mini), McNab (30%), Silken Windhound (30%), English Shepherd (15%), Shetland Sheepdog (15%), English Shepherd (15%), German Shepherd (10%), Herding Breed Cross (10%). Other less affected breeds are: Old English Sheepdog, Border Collie, Berger Blanc Suisse, Bobtail, Wäller. The only way to be sure that a dog is affected or not is to test for it. As more dogs are tested it is likely that the mutation is discovered in other breeds, or that the frequencies change.
Learn more about ivermectin safety.
General safety information for antiparasitics is available in specific articles in this site (click to visit):
- General safety of antiparasitics for domestic animals
- General safety of antiparasitics for humans
- General safety of antiparasitics for the environment
|
WARNING Never use products for livestock on dogs and cats unless they are explicitly approved for both livestock and pets. Pets may not tolerate livestock formulations. Never use agricultural or hygiene products with this or any other active ingredient on livestock or pets, even if there are veterinary products with this same active ingredient approved for use on animals. The formulations for agricultural or hygiene use are different and may be toxic for livestock or pets. It is obvious that veterinary products are not intended for and should never be used on humans!!! |
MARKETING & USAGE
Decade of introduction: 1980
Introduced by: MERCK SHARP & DOHME (→ MERIAL)
Some original brands: IVOMEC, HEARTGARD
Patent: Expired (particular formulations may be still patent-protected)
Use on LIVESTOCK: Massive: ivermectin is the parasiticide most used worldwide
Use in HORSES: Massive
Use in DOGS and CATS: Abundant
Main delivery forms:
- Drenches
- Feed additives
- Injectables
- Oral pastes & gels
- Pour-ons
- Slow-release boluses
- Tablets, pills, etc
Use in human medicine: Yes
Use in public/domestic hygiene: Yes
Use in agriculture: Yes
Generics available: Yes, NUMBERLESS
PARASITE RESISTANCE
- On livestock: Yes, very frequent in gastrointestinal roundworms of sheep, goats and cattle, less frequent in pgs. There are reports on cattle ticks (Boophilus microplus) resistance to ivermectin as well.
- On horses: Yes, in gastrointestinal roundworms such as Small strongyles (Cyathostomins) and Parascaris equorum.
- On pets: Yes, reported in heartworm microfilariae in the USA (particularly in the South).
Visit also the section in this site about parasite resistance to antiparasitics and more specifically to ivermectin.
SPECIFIC FEATURES
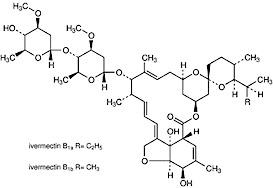
Ivermectin is a mixture of avermectin H2B1a (90%) and avermectin H2B1b (10%).
Both are semi-synthetic derivatives of avermectin B1 and avermectin B2, which are obtained directly from fermentation extracts of Streptomyces avermitilis, and have the same spectrum of activity.
Ivermectin is undoubtedly the most revolutionary parasiticide in modern veterinary medicine, the one with the broadest spectrum of activity, and the most sold. Today's sales of ivermectin products worldwide probably exceed 2 billion US$ at end-user level.
After patent expiry numberless generics have flooded the market, especially for livestock. With very few exceptions most Animal Health laboratories, large and small, local or multinational have added one or more parasiticide brands with ivermectin into their product range. There are thousands of brands with ivermectin, alone or in mixtures.
Efficacy of ivermectin
In livestock ivermectin is effective against the major parasitic roundworms: gastrointestinal (e.g. Haemonchus spp, Cooperia spp, Ostertagia spp, Trichostrongylus spp) and pulmonary (e.g. Dictyocaulus spp). It is also effecive against most mites and lice species, and against numerous myiases (e.g. those caused by screwworm flies, bot flies and warble flies) usually regardless of the delivery form (pour-on, injectable, drench or feed additive).
However, at the usual therapeutic dose, ivermectin products are ineffective against tapeworms and flukes, fleas, mosquitoes, horse and deer flies and stable flies and several other flying insects. Cattle horn flies are only controlled by pour-on formulations. Cattle ticks (Boophilus spp) are only sufficiently controlled by pour-ons and high concentration (≥ 3.15%) injectables, not by the classic 1% injectables, drenches or feed additives. Other ticks (e.g. Amblyomma spp, Hyalomma spp Dermacentor spp, Ixodes spp, Rhipicephalus spp) are not controlled at all by ivermectin products.
In poultry, ivermectin at the therapeutic dose is also ineffective against bloodsucking poultry mites such as red poultry mites (Dermanyssus gallinae), northern fowl mites (Ornithonyssus sylviarum), and tropical fowl mites (Ornithonyssus bursa), as well as against soft ticks (e.g. Argas spp, Ornithodorus spp, Otobius spp, etc.)
In horses, ivermectin is vastly used against gastrointestinal roundworms, e.g. against so-called Small Strogyles (Cyathostomins), Large Strongyles (Strongylus spp), Parascaris equorum, etc. and against horse bots (Gasterophilus spp).
In pets, ivermectin at the therapeutic dose is an effective heartworm preventative (Dirofilaria spp) and controls a few other roundworms as well (e.g. Toxocara canis). The combination with a broad-spectrum nematicide (e.g. pyrantel) controls most pet roundworms (incl. ascarids and hookworms). However, alone or in combination with a nematicide, at the therapeutic dose against heartworm, ivermectin is ineffective against tapeworms, flukes, fleas, ticks, mites, lice and flies.
Pharmacokinetics of ivermectin
Ivermectin is a rather lipophilic molecule. Regardless of the delivery form (topical, oral or injection) it is well absorbed into blood and distributed throughout the host's organism. It tends to be deposited in the body fat and the liver, from where it is progressively released and metabolized. The pharmacokinetic behavior varies for each species and depends strongly on the delivery form and the formulation.
Absorption into blood in cattle and sheep after subcutaneous injection varies with the vehicle. After injection with a lipophilic vehicle absorption is slower than with a hydrophilic one and persistence in the organism is longer. But the blood peak reached is also lower. After oral administration (e.g. drench), absorption into blood is significantly faster, the maximum concentration achieved in blood is also higher, and it is reached earlier than after injection. The consequence is also a shorter residual effect than after injection. In cats and dogs absorption after injection is usually faster than on ruminants.
In the last years so-called long-acting (LA) injectable formulations of ivermectin (or other macrocyclic lactones) have been introduced for ruminants in many countries (e.g. Latin America). They are now very popular and have vastly replaced the slow-release boluses. Many brands contain 3,15% ivermectin, other brands slightly less or even more (up to 4%). The usual dose is 630 mcg/kg bw (instead of 200 mcg/kg bw for the classic 1% formulation). The pharmacokinetic behavior of such LA formulations is similar to the classic 1% formulation. However, the massive higher dose substantially prolongs the residual effect against most parasites, and the higher blood peaks allow higher efficacy against several parasites than the 1% formulation.
After oral administration (mainly to sheep and goats) the type and amount of feed can influence ivermectin's bioavailability. Blood concentrations achieved are lower in grazing sheep than in those fed on hay or concentrate. It is known that ivermectin and its metabolites bind strongly to food particles in the stomach. And food type can significantly influence the time that feed remains in the rumen of ruminants before passing to the abomassum. The faster the food leaves the rumen, the shorter and lower is the absorption. It has been determined that a 50% reduction of food 36 hours prior to and after drenching increases bioavailability of orally administered ivermectin in sheep by about 50%, because it prolongs the time that food remains in the rumen.
Distribution of ivermectin to all organs and most body fluids is sufficient to achieve effective concentrations against the major parasites after both oral, injectable and topical administration. Highest tissue residues are detected in body fat and liver.
Excretion of ivermectin is independent from the delivery form and is achieved to >90% through bile and feces. Only about 2% is excreted through urine. About 45% of the eliminated ivermectin is the parent molecule and the rest are various metabolites. Excretion in goats is significantly faster than in sheep. In sheep it takes about 11 days for ivermectin to drop below the detectable level in blood, whereas in goats this level is reached 4 to 5 days after administration.
Withholding periods for meat vary for each host, delivery form and dosage and are between 3 and 7 weeks for the usual dose (200-300 mcg/kg bw after injection; 500 mcg/kg after pour-on administration). For the LA injectables the withholding period is usually about 4 months. Surprisingly, exactly the same formulation at the same dose and for the same target animal may have significantly different withholding periods in different countries: unfortunately regulatory authorities do not follow the same safety standards everywhere.
In lactating animals about 5% of the administered dose is excreted though the milk. It can be detected in milk already 12 hours after administration. Peak concentrations are achieved aprox. 2 days after administration. Residues in milk remain detectable during aprox. 18 days (after the usual dose of 200 mcg/kg). This is why ivermectin is usually not approved for use on dairy animals whose milk is intended for human consumption.
Injectable formulations of ivermectin are not used on horses. The reason is apparently that, shortly after introduction, it was noticed that horses were more prone to develop severe clostridial infections at the injection site (due to contamination of the needles) and other undesired side effects than cattle or sheep. In addition, the pharmacokinetic behavior of ivermectin on horses is different than in ruminants. For these reasons oral pastes were developed for horses that do not show such side effects.
Mechanism of action of ivermectin
As all macrocyclic lactones, ivermectin acts as agonist of the GABA (gamma-aminobutyric acid) neurotransmitter in nerve cells and also binds to glutamate-gated chloride channels in nerve and muscle cells of invertebrates. In both cases it blocks the transmission of neuronal signals of the parasites, which are either paralyzed and expelled out of the body, or they starve. It also affects the reproduction of some parasites by diminishing oviposition or inducing an abnormal oogenesis.
In mammals the GABA receptors occur only in the central nervous system (CNS), i.e. in the brain and the spinal chord. But mammals have a so-called blood-brain barrier that prevents microscopic objects and large molecules to get into the brain. Consequently macrocyclic lactones are much less toxic to mammals than to the parasites that do not have such a barrier, which allows quite high safety margins for use on livestock and pets. A notable exception to this are those dogs that carry the MDR-1 mutation previously mentioned.
Click here to view the list of all technical summaries of antiparasitic active ingredients in this site.