Nitroscanate is an antiparasitic active ingredient used in veterinary medicine in dogs and cats against internal parasites (roundworms, tapeworms). It is not used against agricultural and household pests. It belongs to the chemical class of the isothiocyanates.
Common name: NITROSCANATE
Type: veterinary medecine
Chemical class: Isothiocyanate
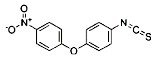
CHEMICAL STRUCTURE
EFFICACY AGAINST PARASITES
Type of action: medium-spectrum nematicide and taenicide anthelmintic, endoparasiticide
Main veterinary parasites controlled: gastrointestinal roundworms (= nematodes), tapeworms (= cestodes)
Efficacy against a specific parasite depends on the delivery form and on the dose administered.
Click here for general information on features and characteristics of PARASITICIDES.
DOSING
Nitroscanate is a broad-spectrum anthelmintic effective against gastrointestinal roundworms and tapeworms in dogs. It is ineffective against flukes and against external parasites. Oral administration is the rule. It is used scarcely in dogs (mainly in the form of tablets). It is not used in cats, livestock or horses.
The table below indicates some usual dosing recommendations for nitroscanate issued by manufacturers or documented in the scientific literature. They may not be approved in some countries.
| Dosing recommendations for NITROSCANATE |
||
| DOGS | ||
| Delivery | Parasites | Dose (against nitroscanate-susceptible parasites) |
| Oral | Gastrointestianl roundworms | ≥50 mg/kg |
| Oral | Tapeworms | ≥50 mg/kg |
DISCLAIMER: Liability is denied for any possible damage or harm to persons, animals or any other goods that could follow the transmission or use of the information, data or recommendations in this site by any site visitor or third parties.
Dosing recommendations for antiparasitics depend on national regulations. National regulatory authorities determine whether a product is approved for a given indication, i.e. use on a particular host at a specific dose and against a specific parasite. Check the labels of the products available in your country for specific information on approved indications.
SAFETY
Oral LD50, rat, acute*: 3503 mg/kg
Dermal LD50, rat, acute*: not found
* These values refer to the active ingredient. Toxicity has to be determined for each formulation as well. Formulations are usually significantly less toxic than the active ingredients.
MRL (maximum residue limit): Not applicable: not approved for livestock
Withholding periods for meat, milk, eggs: Not applicable: not approved for livestock
Learn more about nitroscanate safety (poisoning, intoxication, overdose, antidote, symptoms, etc.).
General information on the safety of veterinary antiparasitics is available in specific articles in this site (click to visit):
- General safety of antiparasitics for domestic animals
- General safety of antiparasitics for humans
- General safety of antiparasitics for the environment
|
WARNING It is obvious that veterinary products are not intended for and should never be used on humans!!! |
MARKETING & USAGE
Decade of introduction: 1970
Introduced by: CIBA-GEIGY → NOVARTIS → ELANCO
Some original brands: LOPATOL
Patent: Expired (particular formulations may be still patent-protected)
Use in LIVESTOCK: No
Use in HORSES: NO
Use in DOGS and CATS: Yes, scarce
Main delivery forms:
Use in human medicine: No
Use in public/domestic hygiene: No
Use in agriculture: No
Generics available: Yes, very few
PARASITE RESISTANCE
In dogs and cats: No
Learn more about parasite resistance and how it develops.
SPECIFIC FEATURES
Nitroscanate is a medium-spectrum anthelmintic used in dogs and cats. It is available in the form of tablets for oral administration. It is not used in livestock.
Efficacy of nitroscanate
Nitroscanate is effective against the major gastrointestinal roundworms (nematodes) of dogs and cats (e.g.Toxocara canis, Toxocara cati, Ancylostoma spp, Uncinaria stenocephala) and tapeworms (e.g. Dipylidium caninum, Echinococcus granulosus, Taenia spp, etc.).
At the therapeutic dose nitroscanate is not effective against whipworms (e.g. Trichuris spp) and flukes (=trematodes).
Nitroscanate has no residual effect. This means that a single administration will kill the parasites present in the host at the time of treatment, but it will not protect the host against re-infestations. To ensure the control of certain parasites re-treatments may be required.
Pharmacokinetics of nitroscanate
After oral administration nitroscanate is poorly absorbed into the bloodstream. Highest plasma levels are reached 24 hours after treatment. Absorption is substantially higher if administered together with food. Excretion is rather fast, mainly through feces and urine in the form of the parent molecule.
Mechanism of action of nitroscanate
The molecular mode of action of nitroscanate has not been elucidated. It is assumed that it acts as an uncoupler of the oxidative phosphorylation in the cell mitochondria, which disturbs the production of ATP, the cellular "fuel". This impairs the parasites motility and probably other processes as well.
Click here to view the list of all technical summaries of antiparasitic active ingredients in this site.