Strongyloides worms, also called threadworms (in the US) or pinworms (in the UK) represent a genus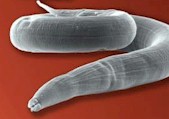 of parasitic roundworms that affects many domestic and wild vertebrates, including horses, cattle, sheep, goats, swine and poultry.
of parasitic roundworms that affects many domestic and wild vertebrates, including horses, cattle, sheep, goats, swine and poultry.
They are found worldwide in regions with a warm und humid climate, mainly in rural areas with poor sanitation standards.
There are several species of veterinary importance for livestock:
- Strongyloides avium. Found worldwide in poultry (chicken, ducks, turkey, etc.).
- Strongyloides papillosus. Found worldwide mainly in cattle, sheep and goats.
- Strongyloides ransomi. Found worldwide in swine and wild boars.
- Strongyloides westeri. Found worldwide in horses, donkeys and other equids.
Strongyloides infections in dogs and cats are handled in a specific article in this site. Some species of this genus (e.g. Strongyloides stercoralis, also a parasite of dogs and cats) are also human parasites in tropical and subtropical regions.
The disease caused by these worms is called strongyloidiasis or strongyloidosis.
Is livestock infected with Strongyloides worms contagious for humans?
NO. The reason is that the species that infect livestock are not human parasites.
You can find additional information in this site on the general biology of parasitic worms and/or roundworms.
Final location of Strongyloides worms
- Predilection site of Strongyloides adults in horses, cattle, sheep, goats and swine is the small intestine. Migrating larvae can be found in skin, blood, lungs and other organs.
- Predilection site of Strongyloides adults in poultry is the cecum.
Anatomy of Strongyloides worms
Adult Strongyloides worms are very small (1 to 6 mm) and thin (about 0.5 mm) – this is why they are called "threadworms" – and almost transparent. Females are longer than males. They have a small mouth capsule.
The worm's body is covered with a cuticle, which is flexible but rather tough. The worms have no external signs of segmentation. They have a tubular digestive system with two openings. Strongyloides worms have a very long esophagus, about 1 third of the whole body length. They also have a nervous system but no excretory organs and no circulatory system, i.e. neither a heart nor blood vessels. Males have chitinous spicules for attaching to the female during copulation.
The adult worms live in the small intestine between the villi (finger-like projections of the wall lining). Sexually active adults are free living outside the hosts, are smaller and their morphology is slightly different from that or parthenogenetic females.
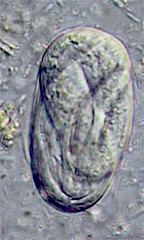
The eggs of livestock and horse species are oval, about 25x50 micrometers and contain a fully developed larva when shed. The eggs of Strongyloides avium are slightly larger, 38x55 micrometers.
of Strongyloides worms
Strongyloides papillosus has a special and complex life cycle. It can complete development both asexually and bisexually.
Inside a final host reproduction is parthenogenetic following a so-called homogonic cycle. This means that the adult females produce viable eggs (up to 2000 a day) asexually, i.e. not fertilized by males but nevertheless capable of developing to adult worms. These eggs contain an already developed L1 larva when deposited.
These asexually produced eggs are shed outside the host with its feces. Once in the environment some of these eggs hatch and develop directly to infective L3 larvae in 2 to 3 days. They can remain infective in the environment for up to 4 months by suitable conditions, but they don't resist cold and dryness. These larvae re-infect a host through the skin or are ingested with contaminated pasture, food or water. After ingestion they undertake a migration through blood vessels, lungs, trachea, mouth and small intestine.
Other larvae develop indirectly, i.e. they follow a bisexual path (a so-called heterogonic cycle) and complete development to adult males or females in the environment, a very uncommon behavior in parasitic helminths. After mating, adult females produce fertilized eggs that develop to infective L3 larvae within 7 to 10 days. These free-living bisexually produced larvae can either complete development to adult males and females in the environment or infect a host (through the skin or orally). Once in the host's gut they complete development to adults but only females are produced, which begin producing eggs parthenogenetically.
Some migrating larvae may not follow the usual path to the lungs but reach other organs, including the placenta, the udders and the milk. This way they can be directly transmitted to the sucklings (galactogenic transmission). Prenatal infection (transplacental/perinatal) is also possible.
The mechanisms that trigger one or the other development paths are not completely elucidated. It seems that the type of host, it's health and especially it's immune system play a role in the future development of the larvae. Medications that affect the immune system (corticosteroids!) may make it easier for Strongyloides infections to become established.
The life cycle of de Strongyloides ransomi in swine is very similar. Sows can be infected with dormant larvae established in body fat. Pregnancy and parturition reactivate these larvae that can infect the piglets through the milk. One week after farrowing the piglets can already start shedding eggs in the feces that become infective only 24 hours later.
Strongyloides westeri of horses has a comparable behavior.
Strongyloides avium has a similar life cycle. It can also infect the birds through the skin.
The prepatent period (time between infection and first eggs shed) is 1 to 3 weeks or longer, depending on the development path, the worm species and the host.
Harm caused by Strongyloides worms, symptoms and diagnosis

Strongyloides papillosus is particularly harmful for calves of up to 6 months of age. The lungs are strongly harmed by migrating larvae, which can also cause secondary infections with bacteria. This results strong coughing, difficult breathing, fever and even pneumonia. In all livestock larvae can also substantially harm the gut's wall, causing serious inflammations (enteritis) and diarrhea (sometimes hemorrhagic), loss of appetite, strong weight losses and even death after massive infections. Anemia can also occur. Larvae migrating through the skin can produce strong dermatitis, with strong itching, especially in the legs.
Strongyloides westeri is also especially harmful for foals. Heavy infections cause severe diarrhea that can be fatal, weakness and weight loss. Larvae migrating through the lungs can cause severe blood loss and various respiratory conditions. Skin penetration can also result in dermatitis. Old animals can support large amounts of worms without clinical symptoms.
Strongyloides ransomi is also particularly harmful for piglets. Massive infections cause hemorrhagic or mucous diarrhea, anemia and even sudden death. Other symptoms include coughing and abdominal pain.
Strongyloides avium is also particularly harmful for young birds. It affects mainly birds kept outdoors. Severe infections cause weakness, weight loss and diarrhea (mucous or hemorrhagic).
Diagnosis is confirmed through detection of small embryonated eggs in the feces. Small larvae (about 600 micrometers long) may be found in elder feces. In poultry adult worms can be found in scrappings of the mucosa of the cecum after necropsy.
Prevention and control of Strongyloides infections
These worms that are quite abundant in warm regions are particularly harmful for young stock. Consequently preventative measures must focus on protecting young stock, which includes protecting pregnant and lactating stock as well, because it can directly infect its offspring.
Strict hygiene and manure removal (in stables, boxes, pens, etc.) is a must, and the facilities should be kept as dry as possible, which diminishes the risk of infection through the skin, since larvae need humidity to survive and reach their hosts. Grazing on dry pastures reduces survival of infective larvae and hinders infection through the skin.
Livestock and horses exposed to these worms often develop natural resistance progressively. Such resistant animals do not become sick if infected, but continue shedding eggs that infect their environment.
Other preventative measures are the same as for all gastrointestinal worms and are explained in a specific article in this site (click here).
Numerous broad spectrum anthelmintics are effective against adult worms and larvae in the gut, e.g. several benzimidazoles (albendazole, febantel, fenbendazole, flubendazole, mebendazole, oxfendazole, etc.), levamisole and pyrantel. However, levamisole, pyrantel and some benzimidazoles are not effective against migrating larvae. Several macrocyclic lactones (e.g. abamectin, doramectin, eprinomectin, ivermectin, moxidectin) are effective against adults, migrating larvae and even dormant larvae.
Depending on the country, most of these anthelmintics are available for oral administration as drenches, feed additives and/or tablets. Levamisole and most macrocyclic lactones are usually also available as injectables. A few active ingredients are also available for livestock as pour-ons and slow-release boluses. For horses most products ara available as oral pastes or geles.
Excepting slow-release boluses, most wormers containing benzimidazoles (e.g. albendazole, febantel, fenbendazole, flubendazole, mebendazole, oxfendazole, etc.) levamisole, tetrahydropyrimidines (e.g. morantel, pyrantel) and other classis anthelmintics kill the worms shortly after treatment and are quickly metabolized and/or excreted within a few hours or days. This means that they have a short residual effect, or no residual effect at all. As a consequence treated animals are cured from worms but do not remain protected against new infections. To ensure that they remain worm-free the animals have to be dewormed periodically, depending on the local epidemiological, ecological and climatic conditions. An exception to this are macrocyclic lactones (e.g. abamectin, doramectin, eprinomectin, ivermectin, moxidectin) and closantel, that offer several weeks protection against re-infestation, depending on the delivery form and the specific parasite.
There are so far no true vaccines against Strongyloides worms, in spite of abundant research on this subject. To learn more about vaccines against parasites of livestock and pets click here.>
Biological control of Strongyloides worms (i.e. using its natural enemies) is so far not feasible. Learn more about biological control of worms.
You may be interested in an article in this site on medicinal plants against external and internal parasites.
Resistance of Strongyloides worms to anthelmintics
There are a few reports on resistance of Strongyloides worms to benzimidazoles and ivermectin in sheep. However, so far resistance of these worms seems to be less widespread than resistance to other gastrointestinal roundworms (e.g. Haemonchus spp, Cooperia spp, Ostertagia spp, etc.).
This means that if an anthelmintic fails to achieve the expected efficacy against Strongyloides worms, there is a certain risk that it is due to resistance to anthelmintics, particularly in sheep, goats and cattle. Nevertheless, it is well known that most cases of product failure are due to incorrect use of a product, or to the use of an unsuited product, not to resistance.
Learn more about parasite resistance and how it develops.
|
Ask your veterinary doctor! If available, follow more specific national or regional recommendations for Strongyloides control. |