Lufenuron is an antiparasitic active ingredient used in veterinary medicine in dogs and cats against fleas. It is also used against agricultural pests. It is a so-called Insect Growth Regulators (IGR) belonging to the chemical class of the benzoylphenyl ureas.
Common name: LUFENURON
Type: veterinary medicine or pesticide, depending un usage
Chemical class: bezoylurea, insect growth regulator
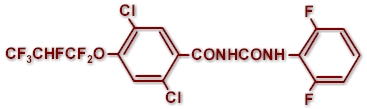
CHEMICAL STRUCTURE
EFFICACY AGAINST PARASITES
Type of action: contact and systemic larvicide
Main veterinary parasites controlled: fleas
Efficacy against a specific parasite depends on the delivery form and on the dose administered.
Click here for general information on features and characteristics of PARASITICIDES.
DOSING
Lufenuron is a systemic insect growth regulator used for flea control in dogs (mainly tablets) and cats (mainly oral suspensions or injectables). It does not kill fleas, but interrupts their development. For this reason it must be used preventively starting at the beginning of the flea season.
It has no effect on ticks, mites or lice. So far it is not used at all in livestock or horses.
The table below indicates some usual dosing recommendations for lufenuron issued by manufacturers or documented in the scientific literature. They may not be approved in some countries.
| Dosing recommendations for LUFENURON |
||
| Delivery | Parasites | Dose (against lufenuron-susceptible parasites) |
| DOGS | ||
| Oral (tablets) | Fleas | 10-30 mg/kg; 4-5 weeks protection |
| CATS | ||
| Injectable | Fleas | 10-20 mg/kg; up to 6 months protection |
| Oral suspension | Fleas | 30-60 mg/kg; 4-5 weeks protection |
DISCLAIMER: Liability is denied for any possible damage or harm to persons, animals or any other goods that could follow the transmission or use of the information, data or recommendations in this site by any site visitor or third parties.
Dosing recommendations for antiparasitics depend on national regulations. National regulatory authorities determine whether a product is approved for a given indication, i.e. use on a particular host at a specific dose and against a specific parasite. Check the labels of the products available in your country for specific information on approved indications.
SAFETY
Oral LD50, rat, acute*: >3000 mg/kg
Dermal LD50, rat, acute*: >4000 mg/kg
* These values refer to the active ingredient. Toxicity has to be determined for each formulation as well. Formulations are usually significantly less toxic than the active ingredients.
MRL (maximum residue limit): Not applicable: not approved for livestock
Withholding periods for meat, milk, eggs: Not applicable: not approved for livestock
Learn more about lufenuron safety (poisoning, intoxication, overdose, antidote, symptoms, etc.).
General safety information for antiparasitics is available in specific articles in this site (click to visit):
- General safety of antiparasitics for domestic animals
- General safety of antiparasitics for humans
- General safety of antiparasitics for the environment
|
WARNING Never use agricultural or hygiene products with this or any other active ingredient on livestock or pets, even if there are veterinary products with this same active ingredient approved for use on animals. The formulations for agricultural or hygiene use are different and may be toxic for livestock or pets. It is obvious that veterinary products are not intended for and should never be used on humans!!! |
MARKETING & USAGE
Decade of introduction: 1990
Introduced by: CIBA-GEIGY (→ NOVARTIS)
Some original brands: PROGRAM, SENTINEL
Patent: Expired (particular formulations may be still patent-protected)
Use in LIVESTOCK: No
Use in HORSES: NO
Use in DOGS and CATS: Yes, moderate
Main delivery forms:
Use in human medicine: No
Use in public/domestic hygiene: No
Use in agriculture: Yes
Generics available: Yes, rather few
SELECTION OF COMMERCIAL BRANDS FOR PETS
- PROGRAM for DOGS and CATS - tablets against FLEAS
- PROGRAM INJECTABLE for CATS - injectable against FLEAS
- PROGRAM ORAL SUSPENSION for CATS - oral suspension against FLEAS
- PROGRAM PLUS for DOGS - tablets against FLEAS, HEARTWORMS and ROUNDWORMS + milbemycin oxime
- SENTINEL for DOGS - tablets against FLEAS, HEARTWORMS and ROUNDWORMS + milbemycin oxime
- SENTINEL SPECTRUM for DOGS - tablets against FLEAS, HEARTWORMS, ROUNDWORMS and TAPEWORMS + milbemycin oxime + praziquantel
PARASITE RESISTANCE
In pets: No
Learn more about parasite resistance and how it develops.
SPECIFIC FEATURES
Lufenuron is an insect development inhibitor belonging to the benzoylureas.
It has a systemic mode of action. After oral administration to dogs and cats it is absorbed to blood and reaches the biting fleas wherever they are in the pet's body.
It is available as tablets for dogs (PROGRAM) and as injectables or oral suspension for cats. There are also mixtures, mainly with wormers. It is not used on livestock.
Efficacy of lufenuron
Lufenuron is highly active against dog and cat fleas, but only as a development inhibitor. This means that adult fleas infesting a pet are not killed by lufenuron, i.e. there is no quick relief. But their eggs will not hatch. This will interrupt the life cycle and the flea population will be decimated. However, there are usually a lot of flea eggs, larvae and nymphs in the pet's environment at the time of treatment, and they will complete development to adult fleas that re-infest the pet. Consequently lufenuron must be used prophylactically (i.e. as a preventative). Treatments should start early in the flea season, when there are only few overwintering immature stages in the environment. The first adult fleas that hatch will infest the pets and lay eggs, but these eggs will not hatch. If lufenuron is administered to pets already highly infested with adult fleas (i.e. therapeutically), either it is used together with a flea adulticide, or it will take about one month for the population to be substantially reduced.
Lufenuron has apparently also antimicotic properties and there are reports on efficacy against certain fungal infections (e.g. Candida albicans) in cats and primates. However there are no commercial products that include such an antimycotic indication.
Pharmacokinetics of lufenuron
Lufenuron is quickly absorbed into blood, both after injection and oral administration. Subsequently it is deposited in the pet's body fat from where it is slowly released back to the bloodstream. This allows maintaining the effective concentration in blood for up to 6 months (after injection). Lufenuron is hardly metabolized. It is slowly eliminated through the liver and the feces.
Mechanism of action of lufenuron
Benzoylureas, as other Chitin Synthesis Inhibitors hamper the synthesis and/or the correct deposit of chitin in the cuticle of insects. As a consequence larvae or nymphs cannot properly molt and die during the molting process. Egg hatching is also interrupted due to the fact that young larvae developing inside the egg have to molt before hatching. If the adult female was treated with a chitin synthesis inhibitor significant amounts of it are passed to the eggs. The embryo can develop normally but it dies during the first molt, still inside the eggshell.
Click here to view the list of all technical summaries of antiparasitic active ingredients in this site.
A personal message
I was very heavily involved in the discovery of lufenuron in the 1990s during my years in NOVARTIS AH. Click here if you want to know more about the discovery and development of lufenuron and PROGRAM.