Lotilaner is a broad-spectrum antiparasitic active ingredient used in veterinary medicine in pets against external parasites (fleas, ticks, lice, mites, etc.). It is not used against agricultural and household pests. It belongs to the chemical class of the isoxazolines.
Common name: LOTILANER
Type: veterinary medicine
Chemical class: ISOXAZOLINES
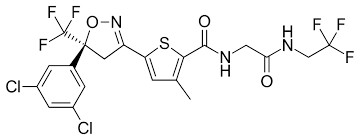
CHEMICAL STRUCTURE
EFFICACY AGAINST PARASITES
Type of action: Broad-spectrum, systemic ectoparasiticide: insecticide, tickicide
Main veterinary parasites controlled: fleas, ticks (Dermacentor reticulatus, Ixodes hexagonus, Ixodes ricinus, Rhipicephalus sanguineus, Dermacentor variabilis; Ixodes scapularis, Amblyomma americanum).
Efficacy against a specific parasite depends on the delivery form and on the dose administered.
Click here for general information on features and characteristics of PARASITICIDES.
DOSING
Lotilaner is an insecticide and acaricide with systemic mode of action that belongs to the chemical class of the isoxazolines, a pesticide class introduced in 2013. So far it is only available for oral administration to dogs (chewable tablets). Lotilaner controls fleas and ticks. It is ineffective against any kind of parasitic worms.
The table below indicates some usual dosing recommendations for lotilaner issued by manufacturers or documented in the scientific literature. They may not be approved in some countries.
| Dosing recommendations for LOTILANER |
||
| DOGS | ||
| Delivery | Parasites | Dose (against lotilaner-susceptible parasites) |
| Oral | Fleas | 20.0-43.8 mg/kg dep on weight ~5 weeks protection |
| Oral | Ticks | 20.0-43.8 mg/kg; dep on weight ~5 weeks protection |
| CATS | ||
| Oral | Fleas | 6.0-53.0 mg/kg; dep on weight ~5 weeks protection |
| Oral | Ticks | 6.0-53.0 mg/kg; dep on weight ~5 weeks protection |
DISCLAIMER: Liability is denied for any possible damage or harm to persons, animals or any other goods that could follow the transmission or use of the information, data or recommendations in this site by any site visitor or third parties.
Dosing recommendations for antiparasitics depend on national regulations. National regulatory authorities determine whether a product is approved for a given indication, i.e. use on a particular host at a specific dose and against a specific parasite. Check the labels of the products available in your country for specific information on approved indications.
SAFETY
Oral LD50, rat, acute*: >2000 mg/kg (source: EMEA)
Dermal LD50, rat, acute*: >2000 mg/kg (source: EMEA)
* These values refer to the active ingredient. Toxicity has to be determined for each formulation as well. Formulations are usually significantly less toxic than the active ingredients.
MRL (maximum residue limit) set for animal tissues: NOT APPLICABLE. Approved only for dogs.
Withholding periods: NOT APPLICABLE. Approved only for dogs.
Target Animal Safety studies done on the tablets approved for dogs indicate that such products are well tolerated by dogs at the therapeutic dose. Beagle dogs treated at up to 5 times the therapeutic dose showed no signs of intoxication.
Due to their recent introduction there is very little knowledge on tolerance in different dog breeds or in young, old or otherwise weak animals.
Since the product is for oral administration, no chemical residues are expected to contaminate the hair-coat of dogs after treatment. Therefore, in contrast with products for external use (e.g. spot-ons) there is no risk of contamination of humans (particularly children) getting in close contact with treated animals.
In September 2018 the FDA of the USA has alerted pet owners and veterinarians about potential neurological adverse events following the use of products containing isoxazolines in dogs. In August 2021 The FDA has extended this alert to cats. Some treated animals have experienced adverse events such as muscle tremors, ataxia (lack of voluntary coordination of muscle movements), and seizures. This regards all products containing isoxazolines. Most treated animals will not show such adverse drug reactions, but some may be affected.
For additional information read the article on lotilaner safety in this site.
General information on the safety of veterinary antiparasitics is available in specific articles in this site (click to visit):
- General safety of antiparasitics for domestic animals
- General safety of antiparasitics for humans
- General safety of antiparasitics for the environment
|
WARNING It is obvious that veterinary products are not intended for and should never be used on humans!!! |
MARKETING & USAGE
Year of introduction: 2017
Introduced by: ELANCO (first described by NOVARTIS)
Some original brands: CREDELIO
Patent: VALID
Use in LIVESTOCK: NO
Use in HORSES: NO
Use in DOGS and CATS: YES
Main delivery forms:
- Chewable tablets
Use in human medicine: No
Use in public/domestic hygiene: No
Use in agriculture: No
Generics available: No
PARASITE RESISTANCE
On pets: No
Learn more about parasite resistance and how it develops.
SPECIFIC FEATURES
Lotilaner is a representative of the isoxazolines, a new class of insecticides discovered in the 2000s. There is so far little information available on this active ingredient. It is similar to other isoxazolines such as afoxolaner, fluralaner or sarolaner. Lotilaner has been approved for use on dogs in the EU in 2017.
Lotilaner is available for oral administration to dogs (CREDELIO), i.e. it has a systemic mode of action. Ingested lotilaner is rapidly absorbed into blood and distributed throughout the whole body of the treated dog. Blood-sucking parasites (mainly fleas and ticks) are killed during their blood meal.
But the systemic mode of action means also that for fleas and/or ticks to be killed, they have to bite the dog first and suck enough blood before the ingested active ingredient kills them.
As other isoxazolines with insecticidal and tickicidal efficacy, lotilaner is a non-competitive GABA (gamma-aminobutyric acid) receptor antagonist, much more selective for GABA receptors in insects or ticks, than for those in mammals, including humans. It binds to chloride channels in nerve and muscle cells, which blocks the transmission of neuronal signals. Affected parasites are paralyzed and die.
Efficacy of lotilaner
CREDELIO from ELANCO was the first product containing lotilaner approved. The EU approval indicates control of fleas (Ctenocephalides felis and C. canis) for at least 5 weeks, and control of ticks (Dermacentor reticulatus, Ixodes hexagonus, Ixodes ricinus, Rhipicephalus sanguineus).
According to the EMEA label full efficacy (>95%) against fleas is achieved within 4 hours of attachment for one month after product administration. Fleas on the animal prior to administration are killed within 6 hours. For adult ticks, the onset of effect (death) is about 48 hours.
Detailed information on the toxicity and the fate of lotilaner in the environment is rather scarce.
Click here to view the list of all technical summaries of antiparasitic active ingredients in this site.